Table of Contents
- Introduction
- Development and Embryology of Urinary System
- Three divisions of Mesoderm:
- RECIPROCAL INDUCTION
- Formation Of Renal Pelvis And Other Structures
- Position of kidney
- Functions of Kidney
- Bladder and Urethra
- Congenital Diseases Of Urinary System
- Conclusion
- Reference
- Read other articles
Introduction
As we all know that nutrition is very essential to human body for its maintenance. Same as that body has to wash out all the waste and toxic substances which are produced after all the metabolic process , this work is done by excretory system also called as urinary system.
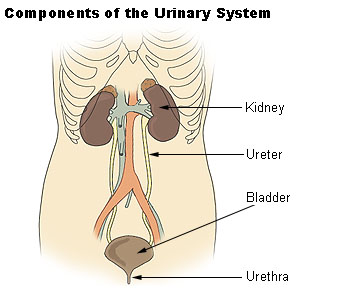
COMPONENTS OF URINARY SYSTEM
- Kidneys
- ureters
- urinary bladder
- urethra
Development and Embryology of Urinary System
This urinary system starts developing at 3rd week of Intrauterine life. This urinary system develops from the mesodermal ridge[Intermediate mesoderm]
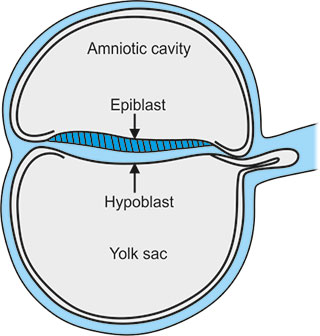
Bilaminar Disc Formation:
The bilaminar disc forms during the 2nd week of embryonic development, following the process of implantation of the blastocyst into the uterine wall.
- Blastocyst: The blastocyst, the stage of the embryo just before implantation, consists of two main components
- Inner Cell Mass (ICM): A cluster of cells that will develop into the embryo.
- Trophoblast: The outer layer of cells that forms part of the placenta.
- Differentiation of the Inner Cell Mass: The inner cell mass of the blastocyst differentiates into two layers
- Epiblast: The upper layer of the inner cell mass, which consists of columnar cells. The epiblast will form the Ectoderm.
- Hypoblast: The lower layer of the inner cell mass, made up of cuboidal cells. The hypoblast will form the Endoderm.
- The amniotic fluid develops above epiblast and yolk sac forms below the hypoblast.
Trilaminar Disc Formation:
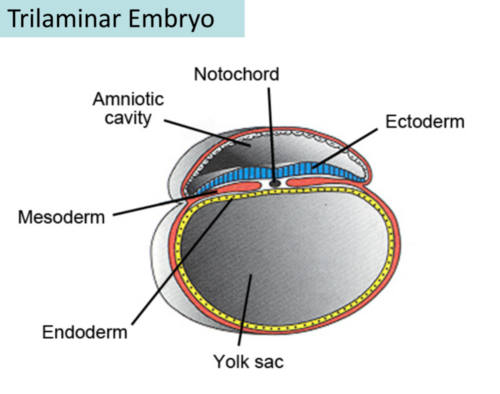
The trilaminar disc forms by the end of the 3rd week of development, during the process of gastrulation. Gastrulation is a crucial event that reorganizes the bilaminar disc into a three-layered structure, which will eventually give rise to all the organs and tissues of the body.
- Gastrulation: During this process, cells from the epiblast layer migrate, and new layers are formed:
- Primitive Streak: This structure appears along the midline of the epiblast, marking the beginning of gastrulation. Cells from the epiblast move toward the streak and ingress (move inward) to form the mesoderm and endoderm layers.
- Formation of the Three Germ Layers:
- Ectoderm: The epiblast cells that remain on the outer surface become the ectoderm, the outermost germ layer.
- Mesoderm: Some epiblast cells ingress through the primitive streak and form the mesoderm, the middle germ layer.
- Endoderm: Some epiblast cells that ingress and move to the inner part of the disc form the endoderm, the innermost germ layer.
- Key Structures Involved in Gastrulation:
- Primitive Streak: The streak marks the midline of the embryo and is crucial for the formation of the mesoderm and endoderm. The cells that ingress through the primitive streak become the mesoderm and endoderm.
- Cloacal Membrane and Oral Membrane: At both ends of the primitive streak, these membranes will give rise to the anus and mouth, respectively.
Three divisions of Mesoderm:
- paraxial mesoderm
- Intermediate mesoderm
- lateral mesoderm
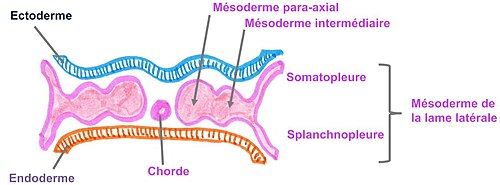
The Urinary System is mainly formed from the Intermediate Mesoderm.
Formation of urinary system starts at week – 4
In 4th week of intra uterine life the intermediate mesoderm starts condensation and formation of nephrogenic cord and urogenital ridge takes place .
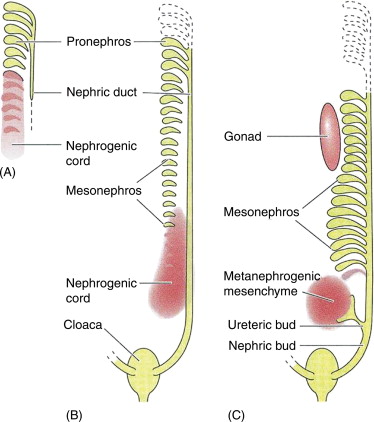
Three overlapping kidney systems are formed from cranial to caudal region during the intra uterine life, they are :
- pronephros
- mesonephros
- metanephros
PRONEPHROS :
These structures formed at the beginning of 4th week and these are rudimentary and non-functional occur in cervical region. These structures represented by 7 to 10 solid cell groups.
These structures form vestigial excretory units called nephrotomes
By the end of 4th week all indications of pronephric system have disappeared .
MESONEPHROS :
These structures are functional but for a short time , occurs in thoraco-lumbar region [upper L3 segments].
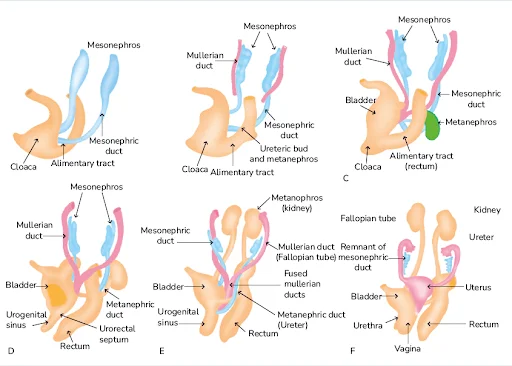
These mesonephros form mesonephric duct also called as wolffian duct and structures arise from this duct are called as mesonephric tubules. These mesonephros acts as primitive urinary system.
Mesonephric duct which formed gets extended into cloaca and the mesonephric tubules form connections with the aorta through angiogenesis.[The process of formation of new blood vessels from existing blood vessel in body]
NOTE : From the aorta the waste gets collected into the mesonephric tubules and from there through mesonephric duct they passed into cloaca .Cloaca is the structure which collects both urine and feces and send them to allantois , this is the structure which get modified into the anus and urinary bladder and urethra in later stage.
These mesonephros works as primitive urinary system from week 5 to week 10 of intra uterine life .
METANEPHROS :
These structures are functional and permanent and forms kidney , these appear at 5th week. These metanephros are formed in pelvic region.
The metanephros starts secreting growth factors which acts on mesonephric duct [wolffian duct] and induce formation of ureteric bud.
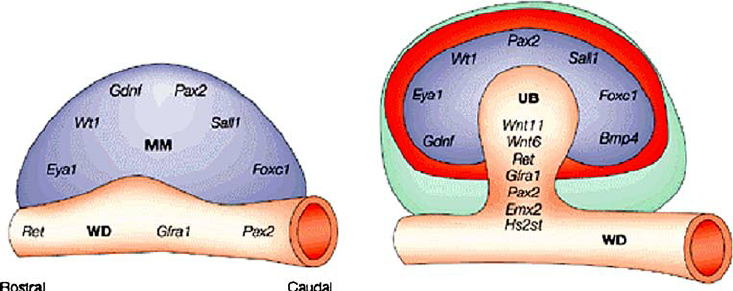
Several important growth factors are involved in the interaction between the metanephros and ureteric bud:
- GDNF (Glial-Derived Neurotrophic Factor):
- Produced by the metanephric mesenchyme (the precursor tissue for the nephron).
- GDNF is a crucial factor for the initial outgrowth of the ureteric bud from the mesonephric duct.
- It acts through the RET receptor on the ureteric bud epithelium, initiating ureteric bud branching.
- GDNF also promotes the survival and proliferation of cells in the ureteric bud and helps in ureteric bud morphogenesis.
- HGF (Hepatocyte Growth Factor):
- Secreted by the metanephric mesenchyme.
- Works in conjunction with GDNF to promote branching morphogenesis of the ureteric bud.
- HGF acts on the ureteric bud epithelium by binding to the c-Met receptor, which activates downstream signaling pathways involved in cell proliferation and migration.
- FGF (Fibroblast Growth Factors):
- Several members of the FGF family (such as FGF1, FGF7, and FGF10) are involved in kidney development.
- These factors are involved in branching morphogenesis of the ureteric bud and in maintaining the mesenchymal-to-epithelial transition in the developing kidney.
- FGFs also help coordinate the growth of the metanephric mesenchyme and regulate nephron formation.
- BMP (Bone Morphogenetic Proteins):
- BMPs, such as BMP4, play an important role in the patterning of the metanephros and regulate the epithelial-to-mesenchymal transition (EMT) of metanephric cells.
- They are involved in the differentiation of the mesenchymal cells into epithelial structures that will form nephrons.
- Wnt Signaling:
- Wnt signaling pathways, including Wnt4 and Wnt9b, are critical for the development of the metanephros.
- Wnt9b, produced by the ureteric bud, is necessary for mesenchymal-to-epithelial transition in the metanephric mesenchyme.
- Wnt4, on the other hand, is involved in nephron differentiation once the mesenchyme has transitioned into epithelial cells.
- Sonic Hedgehog (Shh):
- Sonic Hedgehog signaling, particularly through the Shh pathway, helps regulate the patterning of the metanephros and the maintenance of the nephron progenitor pool.
- It also plays a role in the development of the ureteric bud by coordinating the spatial distribution of mesenchymal cells that interact with the ureteric bud.
RECIPROCAL INDUCTION
The ureteric bud (UB) plays a critical role in kidney development through a process called reciprocal induction, where it interacts with the surrounding metanephric mesenchyme to drive the formation of the functional structures of the kidney. Reciprocal induction refers to the mutual signaling between two different tissues to promote the development of one another. In the case of kidney development, the ureteric bud and metanephric mesenchyme interact in such a way that each tissue influences the development of the other.
Reciprocal induction that works between the ureteric bud and the metanephric mesenchyme:
1. Ureteric Bud Signals to the Metanephric Mesenchyme
The ureteric bud, once it outgrows from the mesonephric duct (Wolffian duct), interacts with the metanephric mesenchyme and induces it to undergo a process called mesenchymal-to-epithelial transition (MET). This is essential for the formation of nephrons .
- Ureteric bud signaling: As the ureteric bud invades the metanephric mesenchyme, it secretes a variety of growth factors, including GDNF (Glial-Derived Neurotrophic Factor), HGF (Hepatocyte Growth Factor), and FGF (Fibroblast Growth Factors). These factors promote the differentiation of the metanephric mesenchyme into epithelial cells, initiating the formation of nephron progenitors.
- Branching morphogenesis: The ureteric bud also stimulates the branching morphogenesis of the collecting duct system by releasing these signaling molecules. These signals guide the bud’s growth and branching within the metanephric mesenchyme.
2. Metanephric Mesenchyme Signals to the Ureteric Bud
In response to signals from the ureteric bud, the metanephric mesenchyme also sends signals back to the ureteric bud to control its growth and branching pattern. This reciprocal signaling is essential for the proper formation of both the nephron and the collecting system.
- Wnt9b signaling: Wnt9b, produced by the metanephric mesenchyme, is one of the most important signals for inducing the ureteric bud to form and branch. It stimulates the ureteric bud epithelium, helping it to undergo proper branching and form the collecting duct system.
- GDNF: The metanephric mesenchyme also produces GDNF, which signals to the ureteric bud via the RET receptor on the epithelial cells of the bud. This signaling is critical for ureteric bud outgrowth and branching. GDNF is one of the first factors to induce the growth of the ureteric bud from the mesonephric duct.
- BMPs (Bone Morphogenetic Proteins): BMPs, which are secreted by the metanephric mesenchyme, influence the differentiation of the ureteric bud cells and the branching of the ureteric tree.
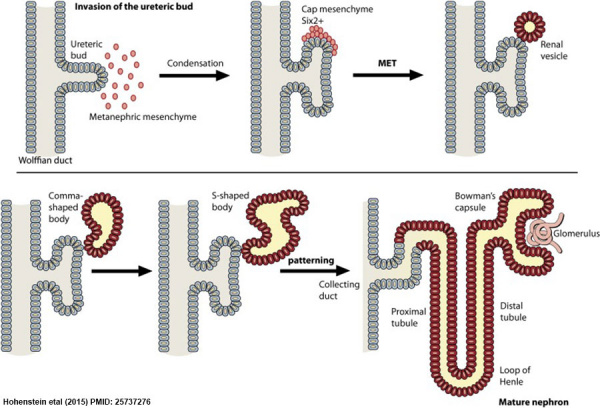
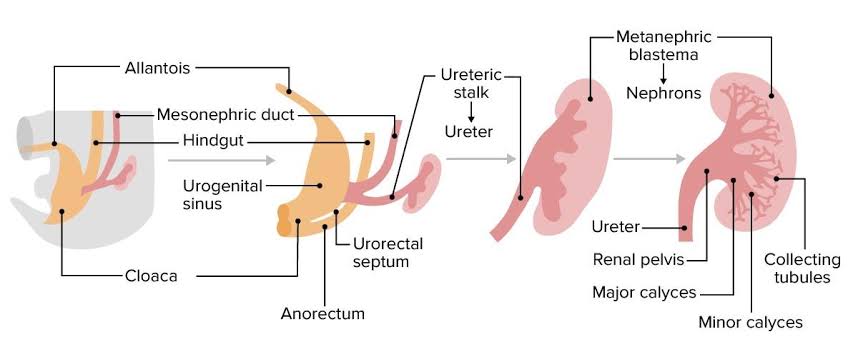
Formation Of Renal Pelvis And Other Structures
The uretic bud which extends into the metanephric blastomere through ureteric stalk forms renal pelvis . Renal pelvis form extensions and formation of major calyces takes place , these major calyces gives rise to minor calyces .At the ends of minor calyces origin of collecting tubules takes place[ approximately 1 to 3 million tubules].
The collecting tubules which are formed release growth factors and helps in condensation of metanephric blastomere at the place where it surrounds the collecting tubule to form metanephric mesodermal cap.
This metanephric mesodermal cap get modified into metanephric vesicle and then into metanephric tubule and get fused with the collecting tubule to form distal convoluted tubule [DCT] , it goes on modifying and form proximal convoluted tubule [PCT] and bowman’s capsule respectively.
The abdominal aorta divides into two branches at the pelvic region into right and left iliac arteries these form tuft of blood capillaries which form glomerular capillaries into bowman’s capsule of the nephron. During this stage the metanephric mesoderm deepens between the PCT and DCT which form loop of Henle.
Hence , kidney develops from 2 sources :
- Metanephric mesoderm : provides excretory units
- Ureteric bud : gives rise to collecting system.
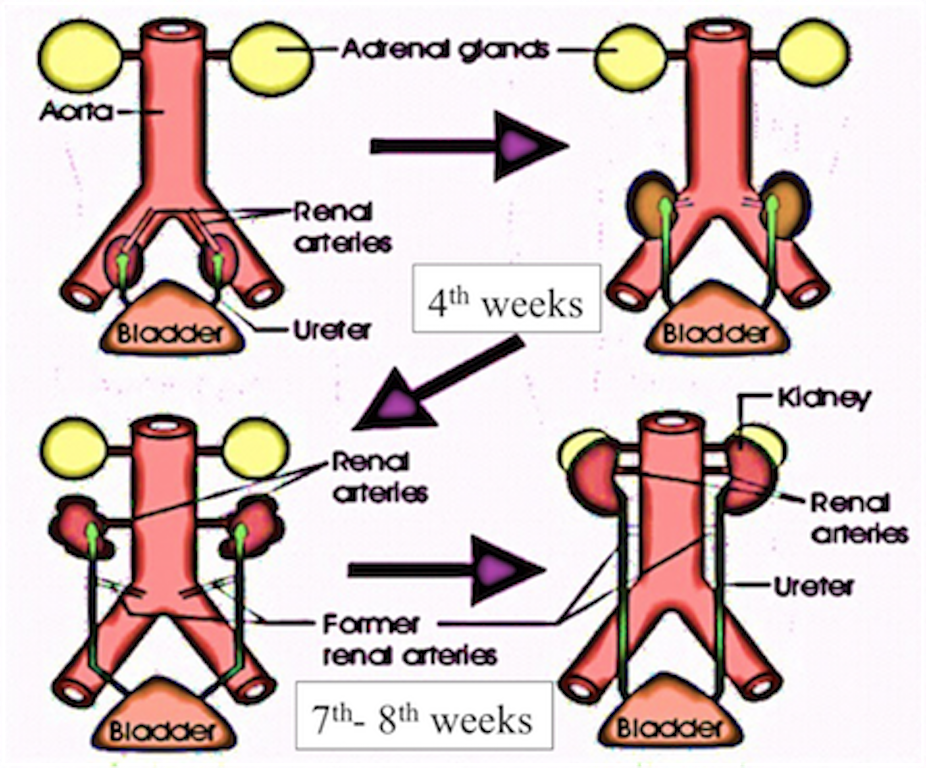
Position of kidney
Initially it is situated in pelvic region later it shifts to more cranial position in the abdomen . In pelvic region the metanephros receive blood supply from the pelvic branch of aorta which gradually gets degenerated . During ascent to abdominal level, it is vascularized by arteries that originated from the aorta at continuously higher levels .The lower vessels gradually get degenerated ,at last kidneys are supplied by renal arteries which are developed lateral branches of aorta.
Functions of Kidney
The metanephros completely becomes functional at week 12 and take over all functions of kidney and mesonephric duct no longer plays any role in formation of urine it takes crucial role in formation of genital tract.
Urine passed into the amniotic cavity and get mixes with the amniotic fluid . The fluid is swallowed by the fetus and recycles through the kidneys.
Note : during fetal life kidneys are not responsible for excretion of waste products because placenta serves this function.
Bladder and Urethra
During 4th to 7th weeks of development cloaca divides into :
- Anteriorly : urogenital sinus
- posteriorly : anal canal
The urorectal septum layer of mesoderm formed between primitive anal canal and the urogenital sinus .
Three portions of urogenital sinus:
- Upper [large] : urinary bladder
- Middle [ pelvic part of urogenital sinus ] : In males it gives rise to prostatic and membranous parts of urethra . In females , female urethra.
- Last part [phallic part] :this part development differs greatly between the two sexes.
The uretic stalk get transformed into ureter and initially bladder continues with the allantois but when the lumen formed into obliterated, thick, fibrous cord the urachus this remains and connects the apex of urinary bladder with the umbilicus . In adults it form median umbilical ligament .
Congenital Diseases Of Urinary System
- Renal agenesis : during birth absence of one or both kidneys due to failure in formation of uretic bud or due failure in fusion of uretic bud into renal mesenchyma [ metanephric blastema]
- Renal hypoplasia : Part of kidney doesn’t develop fully in womb , it may be only slightly smaller or it may tiny than normal kidney , leads to abnormal function.
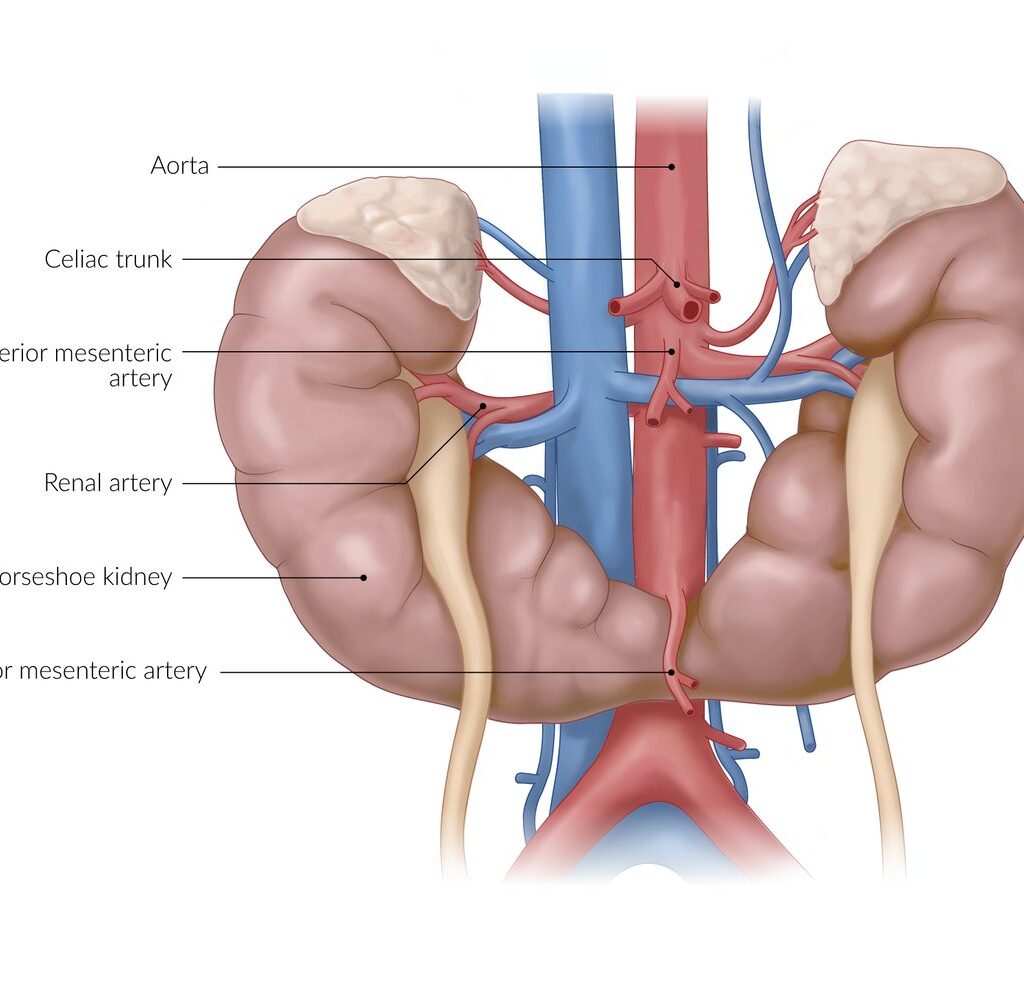
- Horse shoe kidney : It is also called as renal fusion, in this condition the lower ends of both kidneys are fused during fetal stage and form horse shoe like shape .
- polycystic kidney : It is a genetic disorder which causes many fluid filled cysts grow in kidney , these cysts are non cancerous fluid filled sacs.
- Multi cystic dysplastic kidney : In this condition one or both kidneys do not develop normally ,fluid filled sacs [cysts] replace normal kidney tissue which obstructs kidney functions.
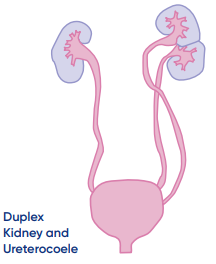
- Duplex Kidney : In this condition kidney have two ureters instead of one .
- Congenital ureteropelvic junction obstruction : Blockage in area where the ureter connects to renal pelvis.
- Ectopic kidney : Mis location of kidney below ,above or on the opposite side than in normal location.
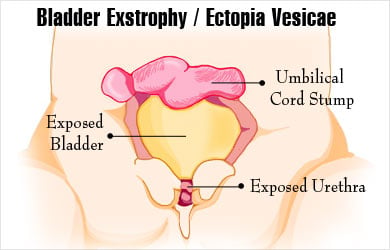
- Exstrophy of Bladder : Rare birth defect where bladder develops outside the fetus, exposed bladder can’t store urine or function normally.
- Posterior urethral valves : obstructive membranes develop in the urethra , it blocks the urine out flow.
- Vesicoureteral Reflex : backward flow of urine from bladder to one or both ureters and sometimes into kidney .
Conclusion
The development of the urinary system is a complex process that begins early in embryogenesis. The kidneys, ureters, bladder, and urethra all derive from different embryological structures: the pronephros, mesonephros, and metanephros, which successively form the functional organs. The formation and differentiation of these structures are crucial for normal renal function, and any disruption during this process can lead to a variety of congenital disorders.
Congenital diseases of the urinary system arise due to abnormal development during embryogenesis, often resulting in malformations like renal agenesis, polycystic kidney disease, duplex kidneys, or abnormalities in the ureters and bladder. These disorders can range from being asymptomatic to causing significant functional impairment, depending on the severity and specific nature of the malformation. Early detection and intervention can greatly improve the outcomes of affected individuals, emphasizing the importance of prenatal screening and ongoing monitoring.
In summary, understanding the embryology of the urinary system is essential for diagnosing and treating congenital diseases. Continued research into the genetic and molecular basis of these conditions can provide better insights for prevention, early detection, and targeted therapies, ultimately enhancing the quality of life for individuals affected by these conditions.
Reference
Langman’s Medical Embryology, 15e T. W. Sadler view
Read other articles
- Global Perspectives on Folliculitis: Ayurveda’s Approach to Prevention, Treatment, and Healthcare Disparities
- How to Lose Weight in 1 Week: Ayurvedic and Research-Based Insights
- 50 Research Paper Insights into Sleep: The Ultimate Guide to Health, Cognitive Performance, and Longevity
- Top 20 Most comely Useful Instruments in Physiology lab with their classification- part 4
- BEST AYURVEDIC DIET AND NUTRITION GUIDE
- AYURVEDA INTRODUCTION
- Growth of the Ayurveda Wellness Market in 2024: Personalization, Technology, and Global Expansion
- Shat Kriyakala: Understanding 6 Stage of Disease Progression in Ayurveda and Its Modern Relevance
- Understanding Concepts 3 Doshas in Ayurveda: Tridosha
- Anatomy, Function, and Clinical Insights into the Mediastinum: A Comprehensive Overview



Pingback: Understanding Of Renal Agenesis : Symptoms , Causes , Diagnosis and Treatment -
Pingback: Gait Abnormalities: Over View On Types of Gait Abnormalities -
Pingback: Over view : Internal Structure of Kidney -